PFAS & Environment
It is easy to look around and feel shocked by the state of our environment. We are bombarded on social media with pictures of marine mammals entangled in discarded fishing nets and we walk down our streets seeing litter building up in our drains. We know the dangers of single-use plastic and marine litter, and we’re aware of the devastating reality of its impact. But what about the problems we can’t see?
We can’t see PFAS molecules spreading throughout our environment, but they are there. We don’t see them wrapping themselves around marine life and we don’t see them spilling out of the stomachs of sea birds, but again, they are there. There is evidence of PFAS harming the brain, reproductive and hormone systems of polar bears. We know some PFAS can harm the immune system, kidney function and liver function of bottlenose dolphins. PFAS can even affect the immune systems of sea otters 66. https://chemtrust.org/wp-content/uploads/PFAS_Brief_CHEMTrust_2019.pdf.
Persistent organic pollutants (POPs) are a name given to some of the most toxic chemicals, known to have harmful effects on human health and the environment. Being classified as a POP means a chemical has been shown to persist for long periods of time, accumulating in wildlife and humans, and passing from one species to the next as it moves through the food chain. These POPs are classified and regulated under a United Nations treaty known as the Stockholm Convention. Being listed as a POP is the last stage in acknowledging a substance as a real and urgent global pollution problem, a problem that needs action that goes well beyond national boundaries. Because of their ability to persist in the environment, POPs can also have long-standing harmful effects that exist long after their production and use has ceased. PCBs are a well-known example of POPs, one of the original ‘Dirty Dozen’ initially addressed by the Stockholm Convention. Despite a near-global ban on PCBs implemented almost 30 years ago, concentrations remain at a level attributed to the continued decline of Orca (Killer Whale) populations worldwide.
The carbon-fluorine bond that typifies PFAS is one of the strongest known to nature. Under typical soil conditions, it can take over a 1000 years for some PFAS to degrade. Both PFOS and PFOA are already listed within the Stockholm convention, with PFHxS currently under consideration. However, there are many more PFAS that are not yet listed that are already known for their environmental persistence. We are simply dealing with a regulatory system that cannot keep up with the pace of chemical innovation.
To ensure we don’t repeat the mistakes of the past, and to safeguard our environment and wildlife for the future, we need to act now. Every day that passes, the environmental burden of these harmful chemicals increases.
How do PFAS enter the environment?
There are no natural sources of PFAS, they are entirely industrially-made. However, their widespread use, combined with their ability to move and persist, means they can now be found pretty much everywhere you look.
PFAS are found in marine animals, seabirds and predators in all parts of the world, from penguins in the South to polar bears in the North 7[18] Bryan Boulanger, John Vargo, Jerald L. Schnoor a, Keri C. Hornbuckle*. Detection of Perfluorooctane Surfactants in Great Lakes Water. 2004.. They have been recorded in our air 8[19] Barber JL, Berger U, Chaemfa C, Huber S, Jahnke A, Temme C, Jones KC. Analysis of per- and polyfluorinated alkyl substances in air samples from Northwest Europe. J Environ Monit 2007;9(6):530-41., water 9, 10[20] Ahrens L, Gerwinski W, Theobald N, Ebinghaus R. Sources of polyfluoroalkyl compounds in the North Sea, Baltic Sea and Norwegian Sea: Evidence from their spatial distribution in surface water. Marine Pollution Bulletin 2010;60(2):255-260.
[21] Yamashita N, Kannan K, Taniyasu S, Horii Y, Petrick G, Gamo T. A global survey of perfluorinated acids in oceans. Marine Pollution Bulletin 2005;51(8):658-668.
, sediment 11, 12[22] Ahrens L, Felizeter S, Ebinghaus R. Spatial distribution of polyfluoroalkyl compounds in seawater of the German Bight. Chemosphere 2009;76(2):179-184.
[23] Zushi Y, Tamada M, Kanai Y, Masunaga S. Time trends of perfluorinated compounds from the sediment core of Tokyo Bay, Japan (1950s-2004). Environ Pollut 2010;158(3):756-63.
, plants 13[13] Muller CE, De Silva AO, Small J, Williamson M, Wang X, Morris A, Katz S, Gamberg M, Muir DC. Biomagnification of perfluorinated compounds in a remote terrestrial food chain: Lichen-Caribou-wolf. Environ Sci Technol 2011;45(20):8665-73. and wildlife 14[25] Magali Houde, ‡, Jonathan W. Martin, Robert J. Letcher, Keith R. Solomon a, Derek C. G. Muir*, ‡. Biological Monitoring of Polyfluoroalkyl Substances: A Review. 2006.. They are found in rain and snow 15[26] Kim S-K, Kannan K. Perfluorinated Acids in Air, Rain, Snow, Surface Runoff, and Lakes: Relative Importance of Pathways to Contamination of Urban Lakes. 2007., groundwater 16[27] Melissa M. Schultz, Douglas F. Barofsky a, Jennifer A. Field*, ‡. Quantitative Determination of Fluorotelomer Sulfonates in Groundwater by LC MS/MS. 2004., tap water 17[28] Ericson I, Domingo JL, Nadal M, Bigas E, Llebaria X, van Bavel B, Lindstrom G. Levels of perfluorinated chemicals in municipal drinking water from Catalonia, Spain: public health implications. Arch Environ Contam Toxicol 2009;57(4):631-8., rivers 18, 19, 20[29] Hansen KJ, Johnson HO, Eldridge JS, Butenhoff JL, Dick LA. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ Sci Technol 2002;36(8):1681-5.
[30] Michael S. McLachlan, Katrin E. Holmström, Margot Reth a, Berger U. Riverine Discharge of Perfluorinated Carboxylates from the European Continent. 2007.
[31] Möller A, Ahrens L, Surm R, Westerveld J, van der Wielen F, Ebinghaus R, de Voogt P. Distribution and sources of polyfluoroalkyl substances (PFAS) in the River Rhine watershed. Environmental Pollution 2010;158(10):3243-3250.
, lakes 21[32] Bryan Boulanger, John Vargo, Jerald L. Schnoor a, Keri C. Hornbuckle*. Detection of Perfluorooctane Surfactants in Great Lakes Water. 2004. and seawater 10, 22, 23[21] Yamashita N, Kannan K, Taniyasu S, Horii Y, Petrick G, Gamo T. A global survey of perfluorinated acids in oceans. Marine Pollution Bulletin 2005;51(8):658-668.
[33] Nobuyoshi Yamashita, Kurunthachalam Kannan, ‡, Sachi Taniyasu, Yuichi Horii, Tsuyoshi Okazawa, Gert Petrick a, Gamo‖ T. Analysis of Perfluorinated Acids at Parts-Per-Quadrillion Levels in Seawater Using Liquid Chromatography-Tandem Mass Spectrometry. 2004.
[34] Yeung LWY, Dassuncao C, Mabury S, Sunderland EM, Zhang X, Lohmann R. Vertical Profiles, Sources, and Transport of PFASs in the Arctic Ocean. Environ Sci Technol 2017;51(12):6735-6744.
. PFAS are in the water we drink and even the breast milk we feed to our children.
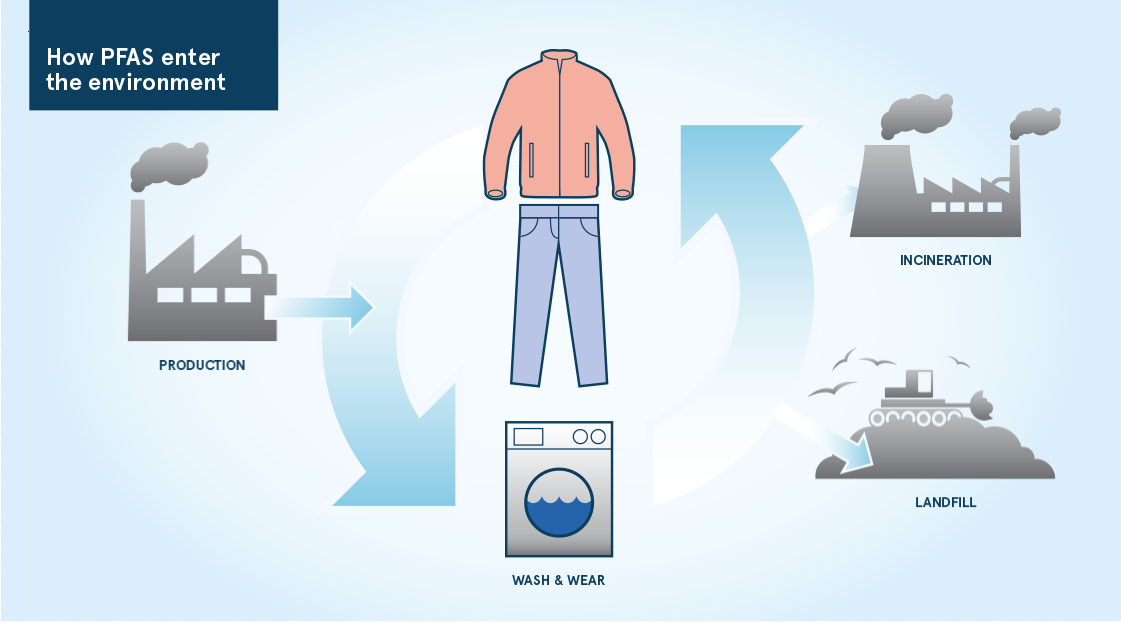
They are lost from chemical plants and manufacturing industries 24[12] Hansen KJ, Johnson HO, Eldridge JS, Butenhoff JL, Dick LA. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ Sci Technol 2002;36(8):1681-5.. They are lost into wastewater and discharged into streams. They can’t be removed by standard water treatment works, so they flow out with the treated water or get applied to our fields as sludge 25[16] Becker AM, Gerstmann S, Frank H. Perfluorooctane surfactants in waste waters, the major source of river pollution. Chemosphere 2008;72(1):115-21.. They are lost during use, sprayed liberally directly into the environment as firefighting foams 26[17] Cheryl A. Moody, Jonathan W. Martin, Wai Chi Kwan, Derek C. G. Muir a, Scott A. Mabury*. Monitoring Perfluorinated Surfactants in Biota and Surface Water Samples Following an Accidental Release of Fire-Fighting Foam into Etobicoke Creek. 2001. or washed from our bike chains with the rain. They are washed off our clothes and they run off our waterproofs 27 [27] Lassen C, Kjølholt J, Hagen Mikkelsen S, Warming M, Jensen AA, Bossi R, Bondgaard Nielsen, Inge. Polyfluoroalkyl substances (PFASs) in textiles for children. Copenhagen2015.. They are emitted from our pans as we leave them to fry on too high a heat and they are absorbed into our food from the packaging it comes in. And when we’re done, they contaminate our compost and they seep out from our landfills 28[28] Busch J, Ahrens L, Sturm R, Ebinghaus R. Polyfluoroalkyl compounds in landfill leachates. Environmental Pollution 2010;158(5):1467-1471..
See image as an example of how one form of PFAS, used to add stain resistant to our clothing, finds its way to our environment:
What happens to PFAS once in the environment?
There are two key characteristics, shared by many of the chemicals within the PFAS group, which make them particularly dangerous once they have entered the environment. PFAS are ‘persistent’, and PFAS are ‘mobile’. This means that once they enter the environment, they stay there for a very long time, i.e. they don’t readily degrade under natural environmental conditions. But they do move. Their mobility means that PFAS can be found far from their original sources. You don’t need to live beside a PFAS factory to be exposed to their effects, hence why we find PFAS in the blood serum of polar bears in the remote arctic. The important implication of this is that if we want to protect ourselves from exposure, we need to cut sources worldwide, not just on our doorstep. Moving manufacturing abroad where regulations are weaker is not a solution, we need joined up global regulations and we as consumers need to care enough to push for change, now, not tomorrow.
When we release PFAS into the air, it circulates around the globe and can be deposited huge distances from the source either by settling out, like dust on an unused surface, or washed out with the rain. If you want to get technical this is ‘long-range atmospheric transport’ combined with either ‘wet or dry deposition’ 29[29] Yeung LWY, Dassuncao C, Mabury S, Sunderland EM, Zhang X, Lohmann R. Vertical Profiles, Sources, and Transport of PFASs in the Arctic Ocean. Environ Sci Technol 2017;51(12):6735-6744.
When we release PFAS into our water, it flows from stream, to river, to sea, circulating in ocean currents. Once it gets into the tiniest of organisms, its position in the food chain simply grows. From plankton, to small fish, to big fish, to sea bird (unless of course we intercept the big fish and take it straight to our plates).
Regardless of how much we know about toxicity, the ease with which these chemicals build up and move, and our complete lack of control once they are out there, is reason enough to demand a precautionary approach to their use. We simply cannot wait for the toxicity studies to catch up, by the time we conclusively prove to industry and regulators the harm scientists already suspect, it is too late.
