The Science
Per- or poly-fluorinated alkyl substances (PFAS) are a group of over 4,700 industrial chemicals, they all share a similar molecular structure, a ‘carbon chain’, and the element, ‘fluorine’. This carbon-fluorine bond is incredibly strong, which makes PFAS extremely difficult to break down.
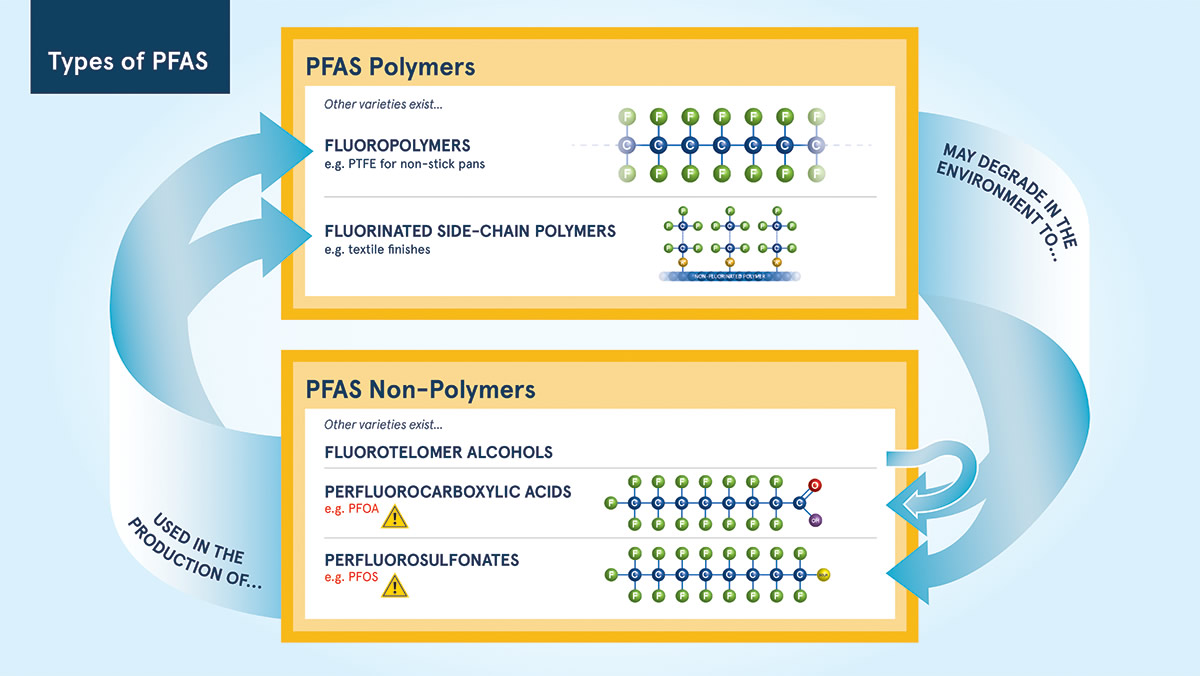
Types of PFAS
PFAS can be split into either polymers or non-polymers. Whilst the terminology might bring back nightmares from school chemistry lessons, the concept is very simple. Poly- means ‘many’ and -mer means ‘segment’, polymers are simply molecules that are long chains made up of many segments. Non-polymers are all the rest.
The non-polymers are also based on chains of carbon atoms, usually with a chain length between 2 and 13 atoms, much shorter than those of polymers. These non-polymers can be split into a further 3 groups. The basic structure of these groups are the same, being primarily made up of carbon and fluorine in a repeating pattern, but the difference is that each group has another chemical group added (either a carboxylic acid, a sulfonic acid or an alcohol). The shorter chain means, compared to polymers, they are more mobile, reactive and more easily transferred into wildlife and humans.
One last distinction, a slightly confusing but essential one as it’s key to how industry is currently dealing with new and existing regulations. The non-polymers usually contain between 2 and 13 atoms making up the ‘chain’ part of the structure. Depending on this chain length, they are referred to as either ‘short-chain’ or ‘long-chain’. When people talk of ‘short-chain’ PFAS they are usually referring to non-polymers where there are 6 or less atoms making up the chain; long-chain refer to non-polymers where there are 7 to 13 atoms. Do not confuse ‘long-chain’ PFAS with polymer PFAS. PFOA and PFOS, are ‘long-chain’ PFAS with 8 atoms (sometimes known as C8 chemistry). Many manufacturers are switching to different versions with only 6 atoms in the chain i.e. C6 chemistry. Evidence is starting to show that these C6 PFAS could be just as persistent and just as toxic as the ones they replace.
PFAS is not a chemical, but a term used to describe a whole group of chemicals which have a similar molecular structure, or ‘carbon chain’, and a specific element, ‘fluorine’. There are more than 3000 types of PFAS commercially available on the world market today.
Polymer PFAS are used on our non-stick pans and clothing
In ‘Fluoropolymers’ the unit that repeats over and over is a simple carbon atom with two fluorine atoms attached; PTFE for non-stick pans is based on fluoropolymers. The slightly more complex ‘Fluorinated side-chain polymers’ are used in textile finishes to give ‘stain resistance’ and ‘water repellent’ qualities.
Fluorinated side-chain polymers start as a basic polymer ‘backbone’ (long chain of atoms), which as the name suggests, has ‘side-chains’ containing fluorine added along its length. The side-chains stick up like the bristles of a comb and act as a barrier towards oil and water. The length of the side-chains, and the nature of the polymer ‘back-bone’ are what gives each individual chemical it’s distinct qualities.
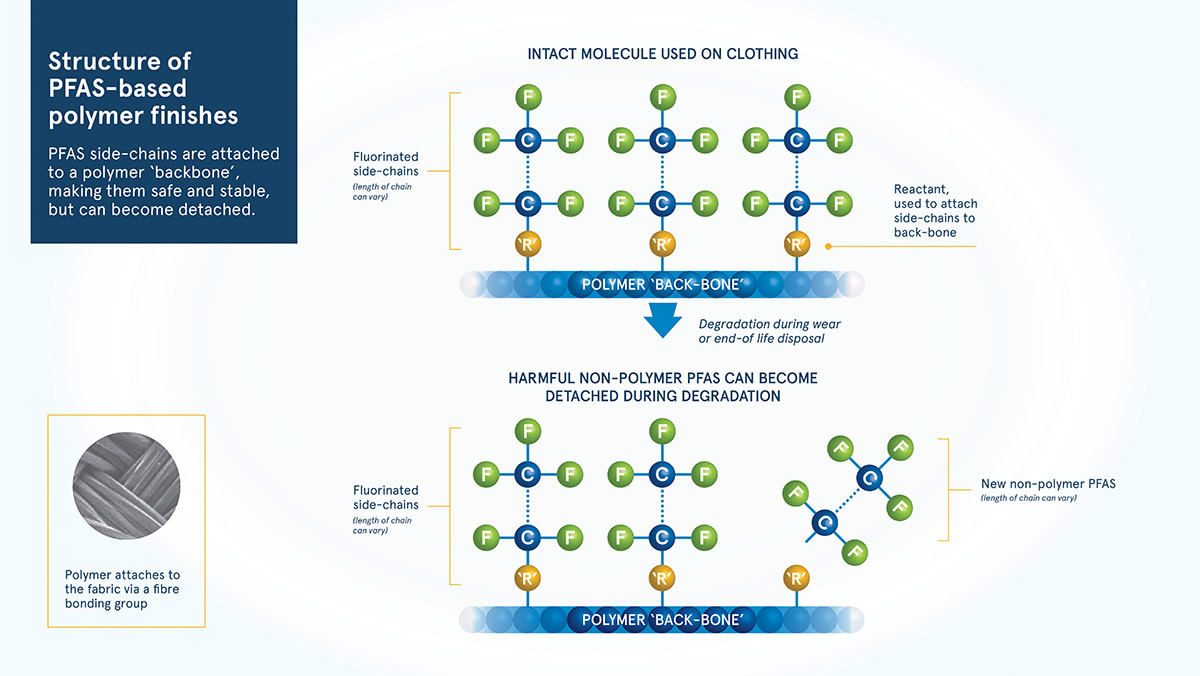
The polymers used to produce textile finishes are not considered to be harmful. This is because polymers are not reactive and the molecules are too large to be easily taken in by the human body (they are not bio-accessible). However, during production and as the polymer begins to break down, harmful non-polymer PFAS can be released into the environment.
POPFREE
We are proud to have participated in POPFREE, an international, multi-stakeholder research project with the goal to find alternatives to PFAS chemistry in many different consumer products.
The 3-year project began in December 2017, organised by a Swedish research consortium, with participants from across Scandinavia, Europe and the world.
The aim of the project was to link up retailer and industry knowledge with state of the art research labs, analysing PFAS-free alternatives by looking at specific requirements of different products. For example, what characteristics are we looking for in stain-resistant school uniforms, compared to stain-resistance in ambulance jackets? As well as looking at textile coatings, the project examined fire-fighting foams, paper coatings, ski waxes and other consumer products. The project included careful analysis of any PFAS-free alternatives, to assess environmental impact and ensure PFAS coatings are replaced with safe alternatives.
Our role in the project was to provide links between POPFREE and UK retailers, carry out consumer surveys and help to share results with industry and the public.

To find out more about the regulations concern PFAS, read our Regulations page: